ChemAxon are a software company that produce a variety of cheminformatics applications and software development modules. A key driver for development has been maintaining portability among various operating systems and a focus on web-based integration thus they have made extensive use of Java. Many of the tools are free to academics or for evaluation and they have a reputation for being very active partners in collaborations.
Whilst ChemAxon have developed a variety of software components I thought I’d start with a review of the chemical editor/viewer Marvin. MarvinView is a Java based chemical viewer for single and multiple chemical structures, queries and reactions, whilst MarvinSketch is a chemical editor for drawing chemical structures, queries and reactions. Both are available as Java applets for embedding into webpages and as Java beans to provide desktop applications.
MarvinSketch
MarvinSketch is provided is a double clickable application and despite being a Java application is not particulalry un-Maclike the scroll bars, icons etc all seem fine whilst in the older versions the keyboard shortcuts all use the control key rather than the “apple” key the latest version now uses the “command” or “apple” key and it fair to say ChemAxon have made great efforts to give a more Maclike look and feel to the application. It is a fairly intuitive application to use since everything is pretty much point and click. The top pallete provides a selection of predrawn templates which the user can click to activate, clicking on the drawing area then draws the selected template, templets can be linked with the pink guides identifying the atoms about to be attached. Atom types can be changed by choosing the required atom from the top pallette or by keyboard input. Keyboard input also provides rapid access to template ph for Phenyl etc. If the template is drawn as an abbrevation e.g. “Ph” for benzene ring they can be expanded to display the full structure by choosing “Expand” from the “Group” menu.
The structures can be moved by selecting all atoms (command-A) and then moving the cursor to the center of the structure a blue square appears and the sttructure can be dragged sideways, move the cursor to edge of the structure and when the blue rotate icon appears the structure can be rotated in 2D. Alternatively after selecting the structure click shift once to move sideways, click shift twice to rotate in 2D or 3-times to rotate in 3D.
The “More” button provides access to a periodic table and also a variety of tools for annotating structures but perhaps more importantly a wide variety of options for building structural queries for database searching. Including the ability to add user defined SMARTS queries. Right click on a structure offers a dropdown menu with a number of options, perhaps the most useful of which is “Copy as SMILES” (SMILES as a simple yet comprehensive chemical language in which molecules and reactions can be specified using ASCII characters representing atom and bond symbols). Marvin supports a variety of different file formats (MOL, MOL2, SDF, RXN, RDF (V2000/V3000), SMILES, SMARTS/SMIRKS (recursive), MRV, InChi, CML, PDB).
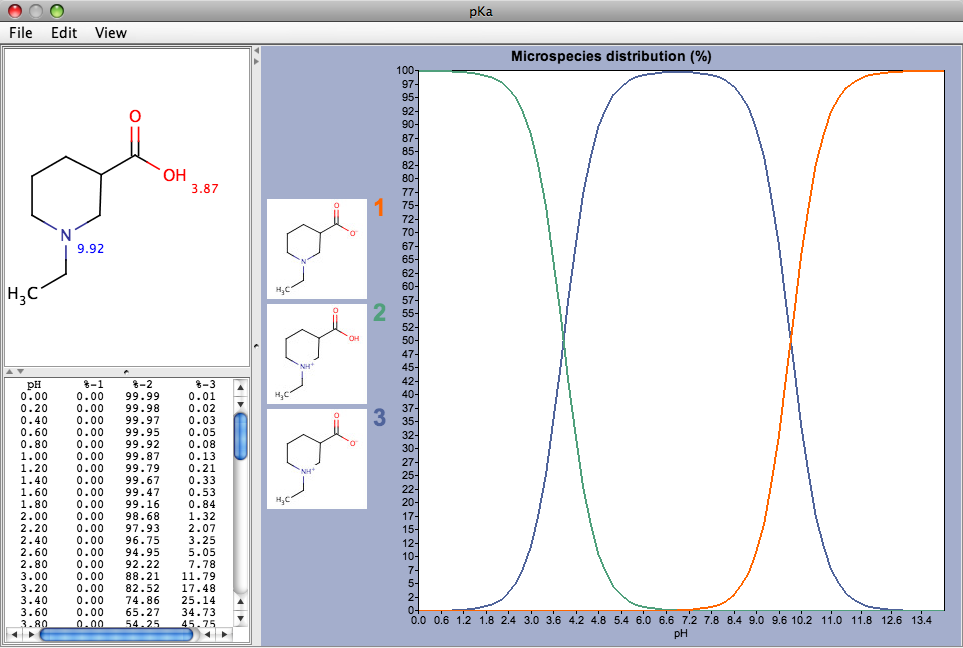
MarvinSketch also provides access to a variety of property calculations by means of a series of dynamically loaded plugins available from the tools menu. These include Elemental Analysis, IUPAC Naming Plugin, pKa Plugin, Major Microspecies Plugin, Isoelectric Point Plugin, Partitioning, logP Plugin, logD Plugin, Charge Plugin, Polarizability Plugin, Orbital Electronegativity Plugin, Tautomerization Plugin, Resonance Plugin, Stereoisomer Plugin, Conformer Plugin, Molecular Dynamics Plugin, Topology Analysis Plugin, Geometry Plugin, Polar Surface Area Plugin (2D), Molecular Surface Area Plugin (3D), Hydrogen Bond Donor-Acceptor Plugin, Huckel Analysis Plugin, Refractivity Plugin. Many of the plugins provide detailed information, for example the pKa plugin dislplays the individual pKas for the ionisable groups and also shows the microspecies distribution curves by pH. The free version allows the calculation of on only a single molecule whereas the commercial versions can be used to calculate any number.
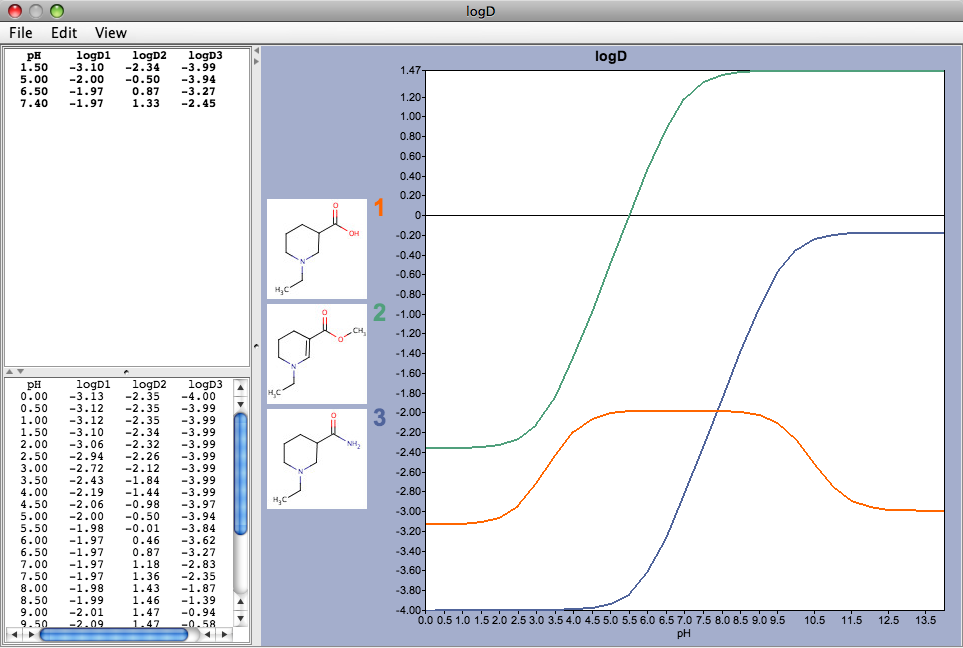
A particularly useful property calculation for drug discovery is the LogD Plugin, below you can see the logD(pH) profiles for a series of compounds and one might use this information to predict the likely sites of absorption from the GI tract as the pH of the varies along the length tract. In fact LogD may well be the most valuable predicted property for drug discovery. The accuracy of the many available tools for chemical property calculations is often debated, for the majority of “regular” chemical structures I’ve found Marvin to be pretty good, however I would strongly suggest that you never undertake a structure activity analysis where you combine values calculated using different packages, choose one and stick to it you are useually interested in the relative values not the absolute number.
The topology analysis plugin provides a wide range of topological descriptors including atom, bond and ring counts, together with a number of distance based indicies. These together with PSA, HBD and HBA provide all of the most commonly used properties for chemiformatics analysis.
A few limitations, MarvinSketch is not a direct replacement for ChemDraw. Whilst MarvinSketch is a great Cheminformtics tool and can be used to build web portals it is not a desktop publishing application, whilst structures can be saved in a variety of image formats and embedded in documents they then of course lose any “chemical” content and cannot be subsequently edited. For writing publication quality manuscripts ChemDraw still sets the standard. If you want to use the clipboard to transfer information between Marvin and other applications the best option is probably to stick to using SMILES.
MarvinView
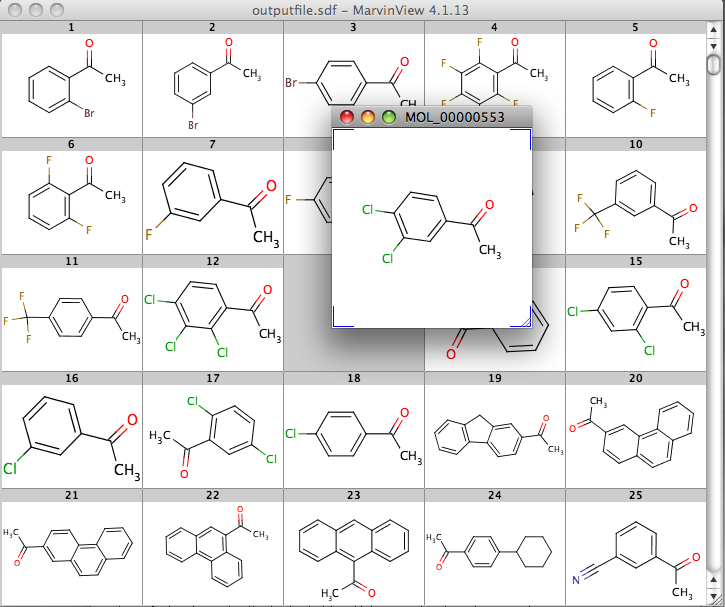
MarvinView is a viewer for single and multiple molecules, and will open and display a variety of file types (MOL, MOL2, SDF, RXN, RDF (V2000 / V3000), SMILES, SMARTS/SMIRKS (recursive), MRV, InChi, CML, PDB). When opening a multimolecule file the structures are displayed in a grid (you can choose the number of structures to display), double click on a structure to see a larger version.
Update to Marvin
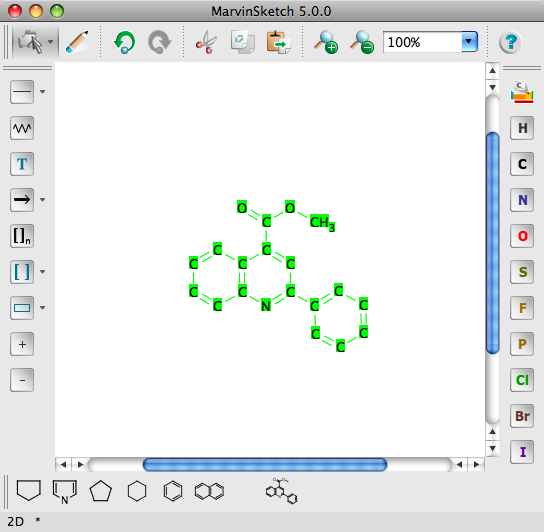
Marvin has just (11 Jan 2008) been updated to version 5.0.0. This brings several improvements to the GUI shown below, I’ve also shown the ability to add your own templates to the templates toolbar at the bottom of the window. The GUI is customisable so you can design your own layout or use one of the four prebuilt layouts designs. Other new features include IUPAC name generation, improvements to the calculation plugins and a new enumerate Markush structures plugin, and several surface area calculations.
Update to Marvin 5.3.4
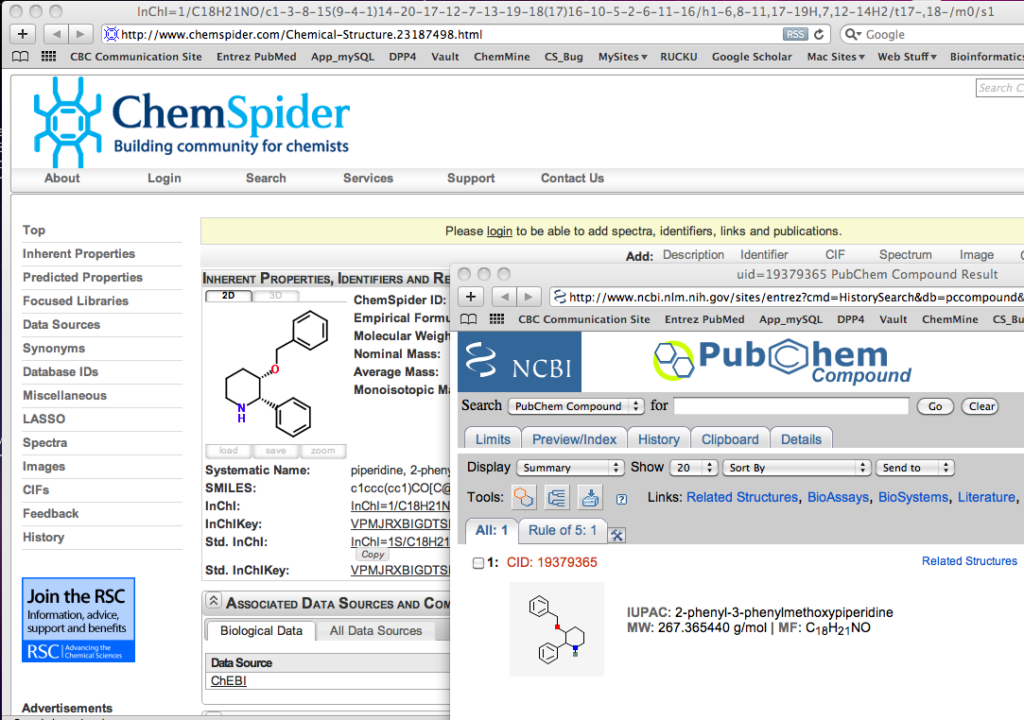
ChemAxon continue to update there applications and I just thought I’d mention this new feature. If you select a structure you get a menu option to search either PubChem or ChemSpider
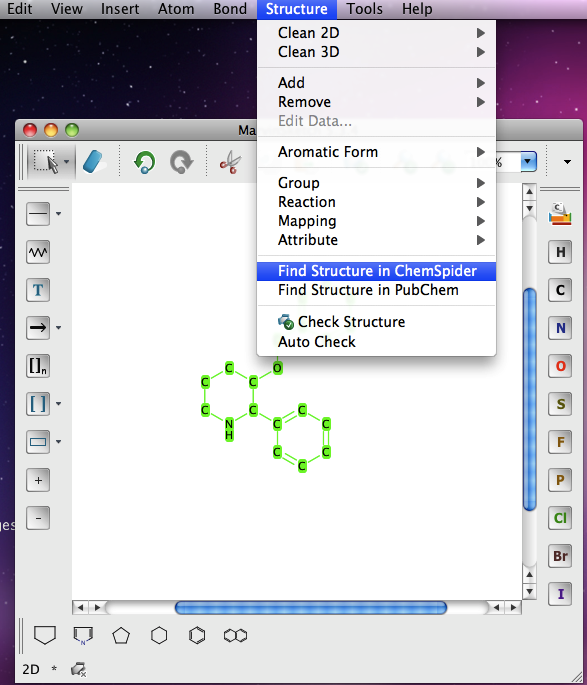
It seems to uses the Inchi to do the search and the results appear in your web browser.

One thought on “A Review of Marvin”
Comments are closed.