Whilst there are a number of chemistry aware spreadsheets Vortex, Stardrop, Datawarrior, InstantJChem, molsoft etc. many people still use Excel or Google sheets. Vexo is
Tag: rdkit
PubChem is an invaluable source of information about 99 million molecules accessible via a website or programmatically. PubChem is an open chemistry database at the National Institutes
TabPFN is a foundation model trained on around 130,000,000 synthetically generated datasets that mimic “real world” tabular data. These datasets sampled dataset size and number
A really interesting preprint caught my attention from Connor Coley’s group at MIT. ShEPhERD diffusing shape, electrostatics, and pharmacophores for bioisosteric drug design https://arxiv.org/abs/2411.04130v1 …

Fabulous blog post from Greg Landrum, Includes a tutorial on installing PostgreSQL and the cartridge with conda. This post shows how to use the RDKit

The 2024.09.1 version of the RDKit was released on the 27th of September and is available via condo-forge (https://anaconda.org/conda-forge/rdkit). Some info on what is new
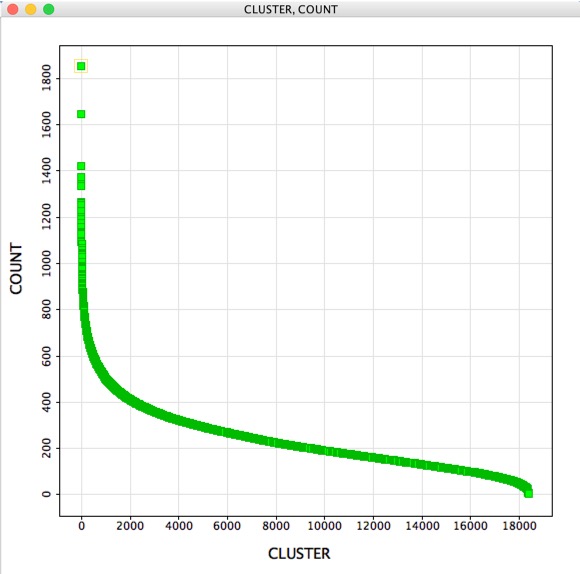
Clustering is an invaluable cheminformatics technique for subdividing a typically large compound collection into small groups of similar compounds. One of the advantages is that
The publication describing lwreg is now available. Here, we present lwreg, a lightweight, yet flexible chemical registration system supporting the capture of both two-dimensional molecular
I first mentioned this utility back in March and I’ve been using it since then and I have to say I find it invaluable. Clipboard-to-SMILES-Converter
rdEditor is a simple RDKit molecule editor GUI using PySide2. Code is on GitHub https://github.com/EBjerrum/rdeditor?tab=readme-ov-file The paper is on chemrxiv https://chemrxiv.org/engage/chemrxiv/article-details/65e6dcfa9138d23161b2979c